AMSBIO는 건강한 가치와 목표를 위해 나아갑니다.

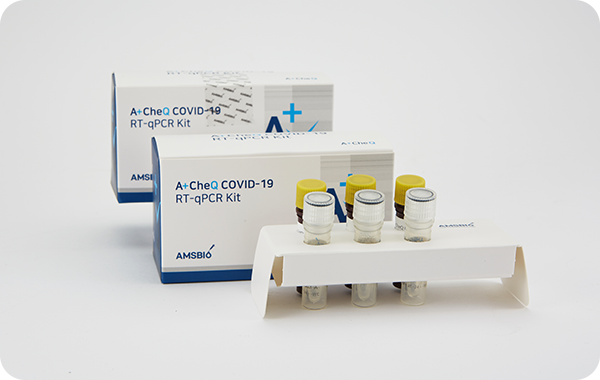
A+CheQ COVID-19 RT-qPCR
Kit RR003
고위험 호흡기 감염병 의심 환자의 검체(구인두 및 비인두 도말물)에서
COVID-19진단에 도움을주며, 정성적 분자진단시약으로 검체에서
추출한 SARS-CoV-2(사스 코로나 바이러스2) 유전자(ORF1ab, N gene)
부위를 실시간역전사효소 연쇄반응법 (Real Time RT-qPCR)을 이용하여
COVID-19 감염진단에 사용되는 체외진단 의료기기이다.

A+CheQ COVID-19 RT-qPCR
Detection Kit RR003
고위험 호흡기 감염병 의심 환자의 검체(구인두 및 비인두 도말물)에서
COVID-19진단에 도움을주며, 정성적 분자진단시약으로 검체에서
추출한 SARS-CoV-2(사스 코로나 바이러스2) 유전자(ORF1ab, N gene)
부위를 실시간역전사효소 연쇄반응법 (Real Time RT-qPCR)을 이용하여
COVID-19 감염진단에 사용되는 체외진단 의료기기이다.

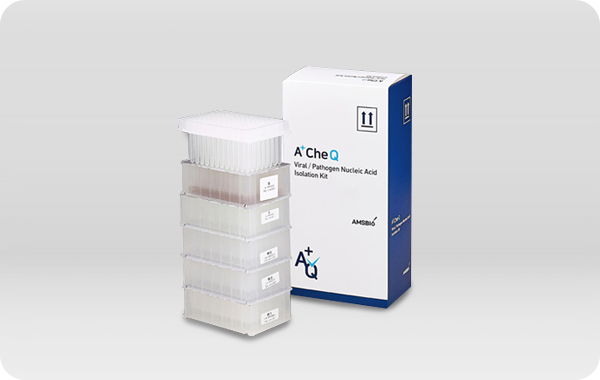
A+CheQ Viral Pathogen Nucleic Acid
Extraction Kit
Lateral Flow immunochromatographic assay 통해 검체에서
SARS-CoV-2의 Nucleocapsid Protein 검체로부터 바이러스 핵산을
추출하는 용도

A+CheQ Viral Pathogen Nucleic Acid
Extraction Kit
Lateral Flow immunochromatographic assay 통해 검체에서
SARS-CoV-2의 Nucleocapsid Protein 검체로부터 바이러스 핵산을
추출하는 용도

- 02-2086-1616
E-Mail. info@amsbio.co.kr
영업시간 AM 09:00 ~ PM 06:00
Support


